You’ve probably heard of uranium, but do you know there’s more than one kind? Two isotopes, U-235 and U-238, dominate the discussion. While they’re similar, one’s a standout in certain applications. Dive deep into the technicalities of these isotopes, understand why U-235’s fission capabilities make it more desirable, and explore the safety factors involved. You’ll quickly see why U-235’s often considered superior.
Understanding Uranium Isotopes
You’ve got to understand that uranium, a radioactive element, has several isotopes, but U-235 and U-238 are the most significant ones. Isotope identification plays a crucial role in uranium mining. U-238 is the most common isotope, making up over 99% of naturally occurring uranium. Yet, it’s not as fissionable as U-235, which is a lot less common but far more valuable in the nuclear industry.
Now, when it comes to uranium mining, you’re essentially looking for pockets of U-235 within large veins of U-238. That’s why isotope identification is so critical. It’s not just about finding uranium; it’s about finding the right kind of uranium. It’s like sifting through sand for gold, only the stakes are much higher.
Uranium mining’s ultimate goal isn’t just to extract this element. It’s to extract the U-235 isotope, which is a Herculean task considering its scarcity. But, once you’ve identified and harvested it, you’ve struck nuclear gold. Its potential in the field of nuclear energy is unparalleled, making the painstaking process of uranium mining and isotope identification downright worth it.
U-235’s Superior Fission Capabilities
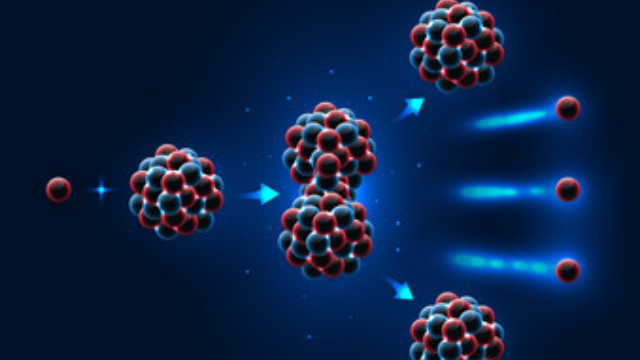
In the world of nuclear energy, it’s U-235’s superior fission capabilities that truly set it apart from U-238. When you look at the fission efficiency comparison, U-235 outperforms U-238 in several key areas.
Let’s break down U-235’s distinct advantages:
- U-235’s energy output is significantly higher. It releases more energy per fission event, making it more efficient.
- U-235 undergoes fission with low-energy neutrons, unlike U-238 which requires high-energy neutrons. This makes U-235 more suitable for nuclear reactors.
- U-235 can sustain a nuclear chain reaction. Once started, it can continue indefinitely under the right conditions.
- The fission of U-235 produces more neutrons which can cause further fissions, leading to a chain reaction. U-238, on the other hand, doesn’t support this process well.
- The byproducts of U-235 fission are less radioactive, reducing long-term waste issues compared to U-238.
U-235 in Nuclear Power Generation
When it comes to generating nuclear power, you’ll find U-235 playing a critical role. It’s the key ingredient in the enrichment process, where natural uranium is processed to increase the concentration of U-235 isotopes.
The majority of nuclear reactor designs require fuel enriched with U-235. It’s not just because of its high fission cross-section; it’s also due to its ability to sustain a nuclear chain reaction. In other words, a neutron can cause a U-235 nucleus to split, releasing energy and more neutrons, which can then cause further fissions. This self-sustaining reaction is the heart of nuclear power generation.
The enrichment process is necessary because naturally occurring uranium contains only about 0.7% of U-235. This is insufficient for most reactor designs, which typically need uranium enriched to between 3% and 5% U-235.
You might wonder why not just use U-238, which is more abundant. Well, U-238 doesn’t easily undergo fission with thermal neutrons, making it less effective for power generation. So, while U-238 is useful in specific applications, it’s U-235 that’s the real workhorse in the world of nuclear power.
Availability of U-235 Vs U-238
Despite U-238 being more prevalent in nature, it’s the scarcity of U-235 that actually makes it more valuable in the realm of nuclear power. U-238 has a natural abundance of 99.28% when compared to U-235’s meager 0.72%. However, the rarity of U-235 doesn’t lessen its importance; rather, it enhances it.
The process of isotope extraction is integral in understanding the availability of these isotopes:
- U-238, while abundant, is not fissile and can’t sustain a nuclear chain reaction on its own.
- U-235, despite its scarcity, is fissile and can sustain a nuclear chain reaction, making it essential in nuclear power generation.
- The extraction of U-235 from natural uranium involves a complex process called enrichment, which increases its concentration.
- Natural uranium’s low U-235 concentration makes the enrichment process expensive and energy-intensive.
- The expense and difficulty associated with the extraction of U-235 highlight its value and importance.
Safety Factors of U-235 Usage
While you might be wondering about the complexities of U-235 extraction, it’s also important to consider the safety factors that come into play when using this isotope in nuclear power generation. The primary concerns revolve around radiation containment and hazard mitigation.
Radiation containment is critical in the use of U-235. You must ensure proper shielding to protect workers and the environment from the harmful effects of radiation. This often involves specific facility designs, robust safety protocols, and state-of-the-art technology to monitor and contain radioactive materials.
Hazard mitigation, on the other hand, involves managing and reducing risks associated with U-235 usage. This includes careful handling and disposal of this isotope to prevent accidents and contamination. It’s crucial to have well-thought-out emergency response plans in place to effectively deal with any mishaps.
Although U-235 is relatively safe when handled correctly, it requires a high level of vigilance due to its inherent radioactivity. So, understanding and implementing these safety factors aren’t just good practices; they’re absolute necessities. As you can see, using U-235 is a delicate balance of harnessing its power while ensuring utmost safety.